Stainless steel grades, composition, molecular structure, production, and properties

In this guide:
- How is stainless steel made?
- What is stainless steel made of?
- Types of stainless steel
- Stainless steel grades
- Is stainless steel magnetic?
- Mechanical properties of stainless steel
- A technical look – stainless steel’s molecular microstructure
- Care and maintenance
Stainless steel is the common name for a large group of ferrous alloys that are resistant to rust. Unlike other iron alloys, stainless steel has a stable passivation layer that protects it from air and moisture. This rust-resistance makes it a good choice for many applications, including outdoor, aqueous, food service, and high-temperature uses.
How is stainless steel made?
Stainless steel can be cast or wrought. The main difference is in how it is formed into a final product. Cast stainless steel is made by pouring liquid metal into a molding container with a specific shape. Wrought stainless steel starts out at a steel mill, where continuous casters make stainless into ingots, blooms, billets, or slabs. These raw manufacturing materials must then be shaped by further work. They are reheated and reworked using rolling or hammering techniques.
Wrought stainless steel products are more common than cast stainless steel products.
Cast stainless steel objects are usually made and finished either in a foundry or with the foundry’s supervision. If they are a small component of a larger product the casting may go to other factories for assembly. Wrought stainless steel starts at a steel mill but is turned into the final product at another factory.

What is stainless steel made of?
Like all steel, stainless steel starts out with a mixture of iron and carbon. What sets this family of alloys apart is that stainless also has a minimum of 10.5% chromium. This element gives stainless steel its characteristic resistance to oxidation. When stainless steel is exposed to the atmosphere, the chromium combines with oxygen to form a thin, stable passivation layer of chromium (III) oxide (Cr2O3). The passivation layer protects the interior steel from oxidization, and quickly reforms if the surface is scratched.
This passivation layer is different than plating. Some metals are plated with zinc, chromium, or nickel, for surface protection. In those cases, the benefits of the coating are lost once a scratch penetrates the plating. The chromium inside stainless steel provides more than this surface protection. It creates its passive film whenever it is exposed to air. Therefore, even if stainless is deeply scratched, the passivation layer will self-heal.
Types of stainless steel
There are several “families” of stainless steel. Each of these families has different proportions of iron, chromium, and carbon. Some have other elements, like nickel, molybdenum, manganese, or copper. The properties of these steels vary by content, making this a versatile group of alloys.
Stainless steel grades
Grades give a hint as to the family of a particular stainless steel. The most common grades are:
- Ferritic stainless: 430, 444, 409
- Austenitic stainless: 304, 302, 303, 310, 316, 317, 321, 347
- Martensitic stainless: 420, 431, 440, 416
- Duplex stainless: 2304, 2205
Sometimes, engineers choose between alloys in the same family, as in the two popular commercial grades of austenitic stainless steel, 304 vs. 316. However, this isn’t always the case. Automotive exhaust systems often choose between 304 and 409. Barbeque grills might be found made of 304 or 430.
Is stainless steel magnetic?
Determining whether a metal is stainless steel through a simple magnet test isn’t definitive. The magnetic properties of stainless steel vary based on the specific grade and composition. While austenitic stainless steel grades (such as those in the 3xx series) are non-magnetic due to their unique microstructures, martensitic, ferritic stainless grades like 430, and duplex steels, which combine austenitic and ferritic properties, tend to exhibit magnetic properties to varying degrees.
Mechanical properties of stainless steel
Stainless steel is usually chosen because it is resistant against corrosion—but it is also chosen because it is steel. Properties such as strength, yield, toughness, hardness, response to work hardening, weldability, and heat-tolerance make steel an incredibly useful metal in engineering, construction, and manufacturing, especially given its cost. An engineer considers the working load and atmospheric conditions of the stainless steel before deciding on a grade.
Tensile properties
Tensile properties of metals are measured by pulling. A representative tensile bar is subjected to pulling force, also known as tensile loading. Upon failure, the tensile strength, yield strength, elongation, and reduction of area are measured.
Hardness
Hardness is the ability of steel to resist indentation and abrasion. The two most common hardness tests are Brinell and Rockwell. In the Brinell test, a small hardened steel ball is forced into the steel by a standard load, and the diameter of the resulting impression is measured. The Rockwell test measures the depth of the indentation. Hardness can be increased in some metals by cold-working, also known as work-hardening. In some metals, hardness can be increased through heat treatment.
Toughness
Toughness is the capacity of steel to yield plastically under very localized stress. A tough steel is resistant to cracking, making toughness a highly desirable quality used in engineering applications. The level of toughness is determined using a dynamic test. A sample bar is notched to localize the stress, then struck by a swinging pendulum. The energy absorbed in breaking the sample bar is measured by how much energy the pendulum loses. Tough metals absorb more energy, while brittle metals absorb less.

Ferritic
Ferritic stainless steels contain iron, carbon, and 10.5–18% chromium. They may contain other alloying elements such as molybdenum or aluminum, but usually in very small amounts. They have a body-centered-cubic (BCC) crystal structure—the same as pure iron at ambient temperature.
Due to their crystal structure, ferritic stainless steels are magnetic. Their relatively low carbon content produces correspondingly low strength. Other weaknesses of the ferritic type include poor weldability and reduced corrosion resistance. They are, however, desirable for engineering applications because of their superior toughness. Ferritic stainless steels are often used for vehicle exhaust pipes, fuel lines, and architectural trim.

Austenitic
Austenitic stainless steels have a face-centered cubic (FCC) crystal structure and are composed of iron, carbon, chromium, and at least 8% nickel. Due to their high chromium and nickel content, they are highly corrosion resistant and non-magnetic. Like ferritic stainless steels, austenitic stainless steels cannot be hardened by heat treatment. However, they can be hardened by cold working. The high nickel content in austenitic stainless steels makes them capable of functioning well in low-temperature applications.
The two most common stainless steels—304 and 316—are both austenitic grades. The primary driver behind the popularity of austenitic stainless steels is the ease with which they can be formed and welded, making them ideal for high-efficiency manufacturing. There are many sub-groups of austenitic stainless steels with wide variations in carbon content. The properties are further tuned by the addition of alloying elements such as molybdenum, titanium, and copper. Austenitic stainless steels are frequently used to produce kitchen sinks, window frames, food processing equipment, and chemical tanks. They are also commonly used for outdoor site furnishings such as benches, stainless steel bollards and bike racks.

Martensitic
Martensitic stainless steels have a body-centered tetragonal (BCT) structure. They contain 12–18% chromium, and have a higher carbon content (0.1–1.2%) than austenitic or ferritic stainless steels. Like the ferritic BCC structure, BCT is magnetic. Martensitic stainless steels are highly useful in situations where the strength of the steel is more important than its weldability or corrosion resistance. The major distinction is that martensitic stainless steel can be hardened by heat treatment because of their high carbon content. This makes them useful for a number of applications including aerospace parts, cutlery, and blades.

Duplex
Duplex stainless steels are the newest stainless steel type. They contain more chromium (19–32%) and molybdenum (up to 5%) than austenitic stainless steels, but significantly less nickel. Duplex stainless steels are sometimes referred to as austenitic-ferritic because they have a hybrid ferritic and austenitic crystalline structure. The roughly half-and-half mix of austenitic and ferritic phases in duplex stainless steels gives it unique advantages. They are more resistant to stress-corrosion cracking than austenitic grades, tougher than ferritic grades, and roughly two times stronger than a pure form of either. The key advantage of duplex stainless steels is corrosion resistance equal to, or exceeding, austenitic grades in the case of chloride exposure.
Duplex stainless steels are also very cost effective. The strength and corrosion resistance of duplex stainless steel are achieved with a lower alloy content than equivalent austenitic grades. Duplex stainless steels are regularly used to produce parts for chloride-exposed applications in the desalination and petrochemical industry. They are also used in the building and construction industries for bridges, pressure vessels, and tie bars.

Precipitation hardening
Precipitation hardening stainless steels can have a range of crystalline structures, however, they all contain both chromium and nickel. Their common characteristics are corrosion resistance, ease of fabrication, and extremely high tensile strength with low-temperature heat treatment.
Austenitic precipitation hardening alloys have mostly been replaced by higher strength superalloys. However, semi-austenitic precipitation hardening stainless steels continue to be used in aerospace applications, and even applied to new forms. Martensitic precipitation hardening stainless steels are stronger than regular martensitic grades and frequently used to produce bars, rods, and wires.
A technical look: stainless steel’s molecular microstructure
When metals freeze out of the molten state, they crystallize and form grains. This crystal structure determines many of the metal’s mechanical properties. Many factors influence this microstructure.
The types of atoms in an alloy change the structure due to the molecules formed by those atomic types. Percentages of each material also determine what arrangements the atoms form.
Temperature has a profound effect on the shape of a metal’s crystal lattice. Different structures begin to form at specific temperatures. Alloys have phase tables that demonstrate what types of grains are common at different temperatures and with different percentages of important elements.
Our iron-carbon phase diagram illustrates the way temperature and carbon affect the formation of grains in steel. It shows three phases of iron formation:
- Ferrite, or alpha iron, (α) is the standard grain formed at temperatures below 912°C.
- Austenite, or gamma iron, (γ), has more densely-packed grain crystals and appears between 912–1394°C.
- Delta iron (δ) forms at heats above 1395°C, before iron turns to liquid at 1538°C. The delta iron phase more closely resembles α-iron, or ferrite.
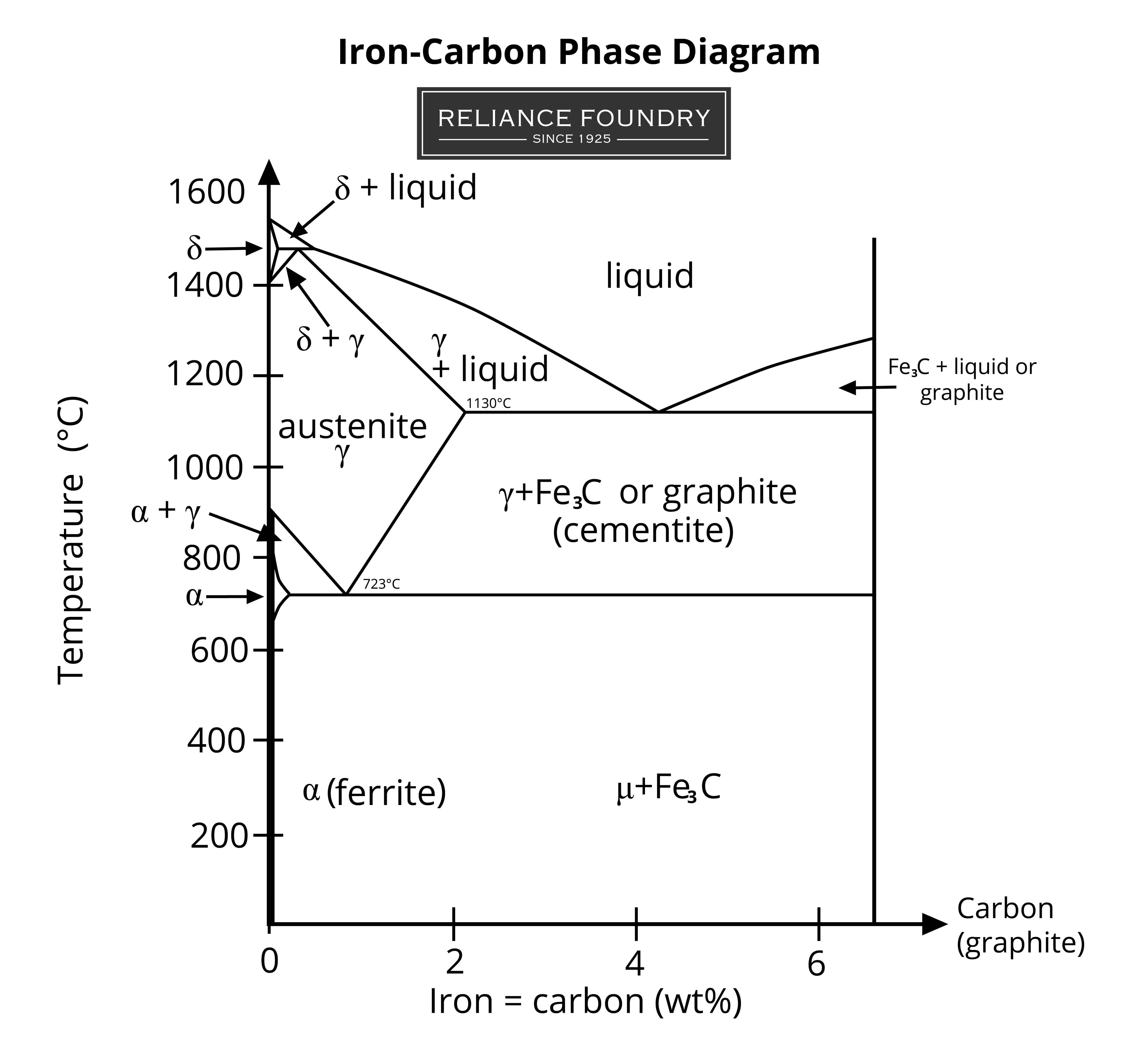
The addition of carbon influences how the basic grains of steel crystallize, stabilize, and interact with one another. Temperature influences how the carbon is absorbed. The high-heat austenite phase is saturated with the carbon, with densely packed molecules of metal. At other temperatures, all the carbon is not absorbed. It creates other molecular structures. For example, it is common for an iron-carbon alloy to contain Fe3C cementite molecules. In pure form, cementite is classified as a ceramic: it is hard and brittle, and it lends these attributes to the final metal. Graphite can also form at a molecular level. The shape of this graphite can affect how the metal behaves when struck. Round graphite nodules can slide past each other when they are hit, deforming but not snapping. In comparison, a metal with a lot of flaky graphite can shear along flake boundaries when struck. How quickly a metal is cooled, and whether it is heat treated or worked, also affect grain size and shape.
Austenitic steels are those that have an austenite lattice with γ-iron. On the iron-carbon phase diagram this lattice is normally found at high temperatures. However, adding nickel and/or manganese allows austenite to remain while the steel cools. The austenite microstructure is known as “face-centered cubic.” Face-centered cubic molecules lend particular properties to metal.
Body-Centered vs. Face-Centered cubic microstructures
Metal is a crystal made of a molecular lattice. Each cell of a lattice is made up of atoms. The number of atoms in each lattice cell, and how they connect with each other, change how this lattice behaves under strain. The basic lattices are primitive, body-centered, and face-centered.
BASIC CELL SHAPES
Primitive Cubic
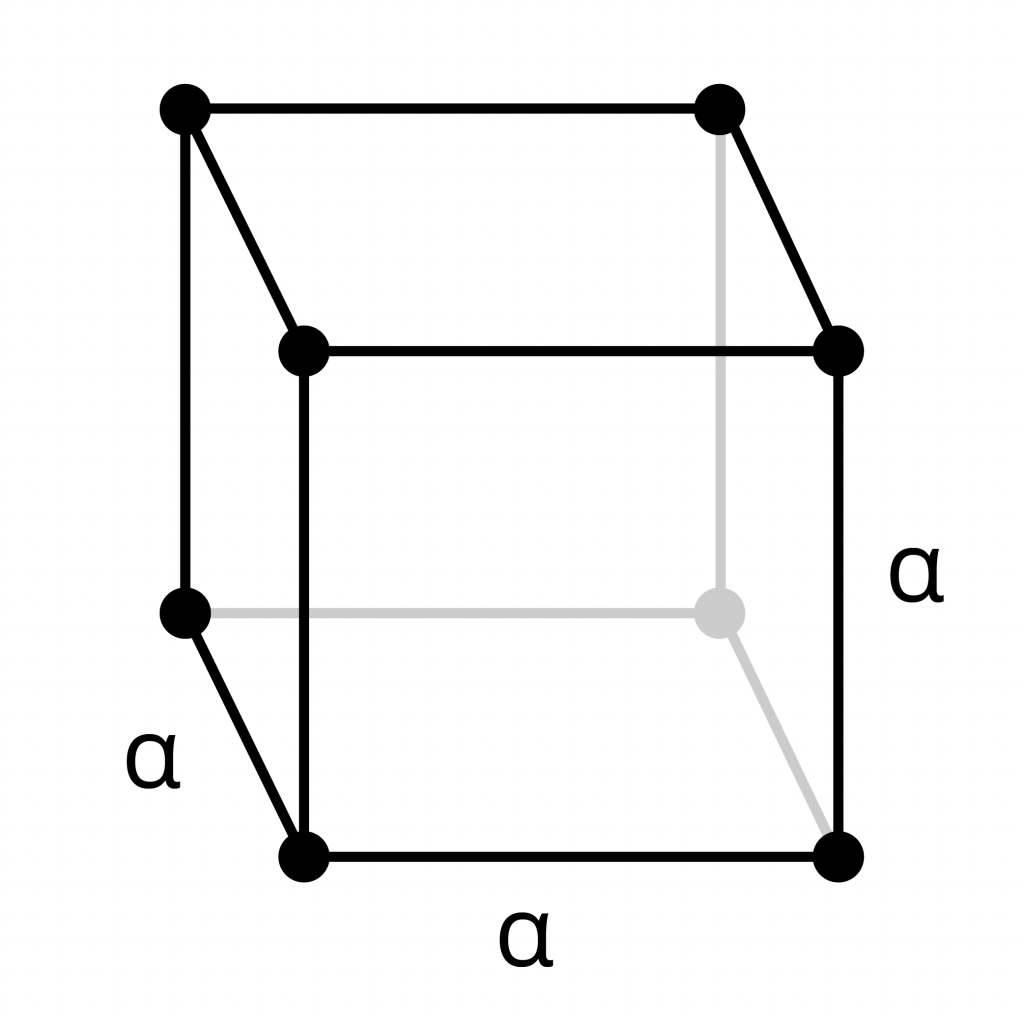
- Each atom in this primitive cube sits at a corner of the cell. Each atom is a connection point in the lattice.
- Each corner atom is shared equally with the cells around it. Therefore, each atom is part of eight adjacent cubes.
- The unit cell contains 1 atom in total. Because each of the corner atoms is shared with eight adjacent cubes, only 1/8 of each atom is inside the primitive cell.
8 x 1/8th piece of each corner atom = 1 atom total.
Body- Centered Cubic (BCC)
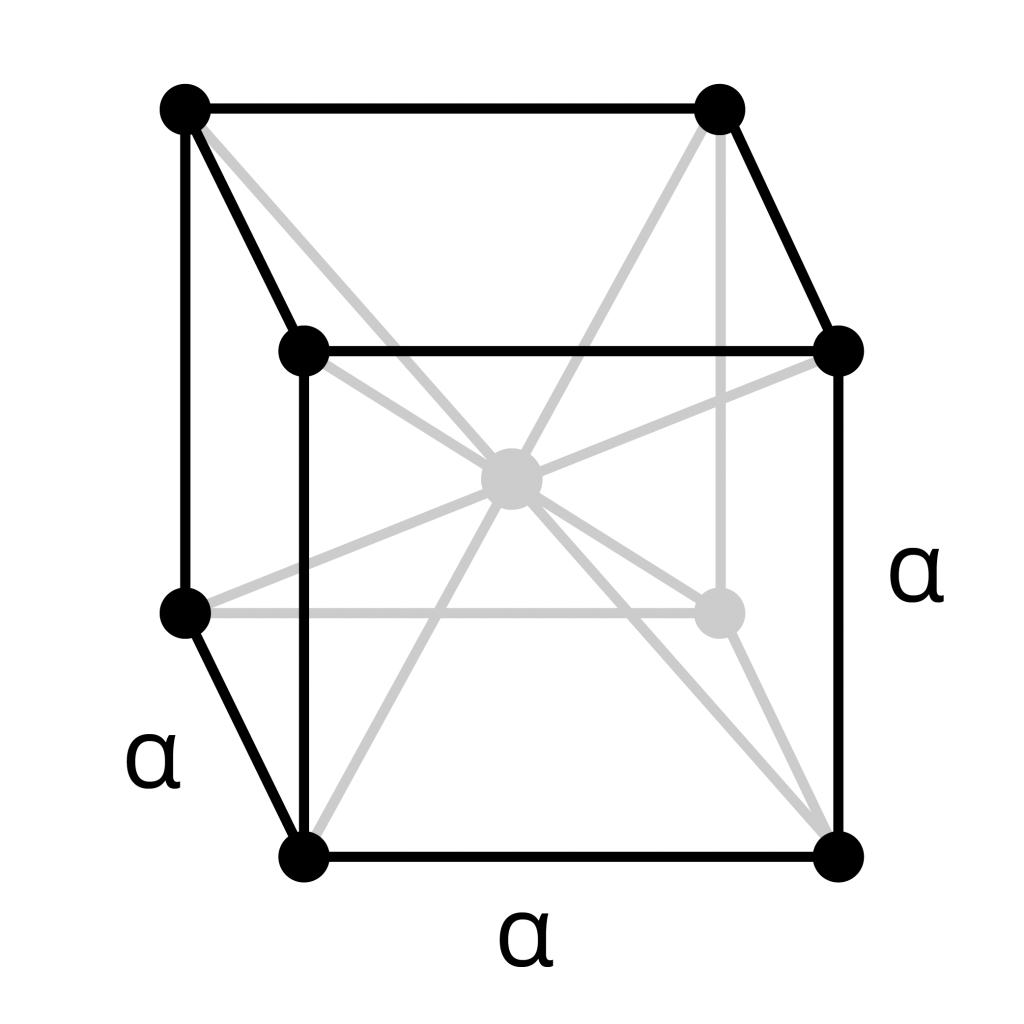
- Like the primitive cubic form, there are atoms at each corner of the cell.
- Additionally, an atom sits in the middle of the cell. This atom is shared by no other cells: there are 8 cells joined to the lattice and one only to the atom.
- The unit cell contains 2 atoms in total:
8 atoms x 1/8 share per atom, as in the primitive cubic structure, plus the atom in the center. - Alpha-iron (ferrite) and delta-iron are both BCC metals.
Face-Centered Cubic (FCC)
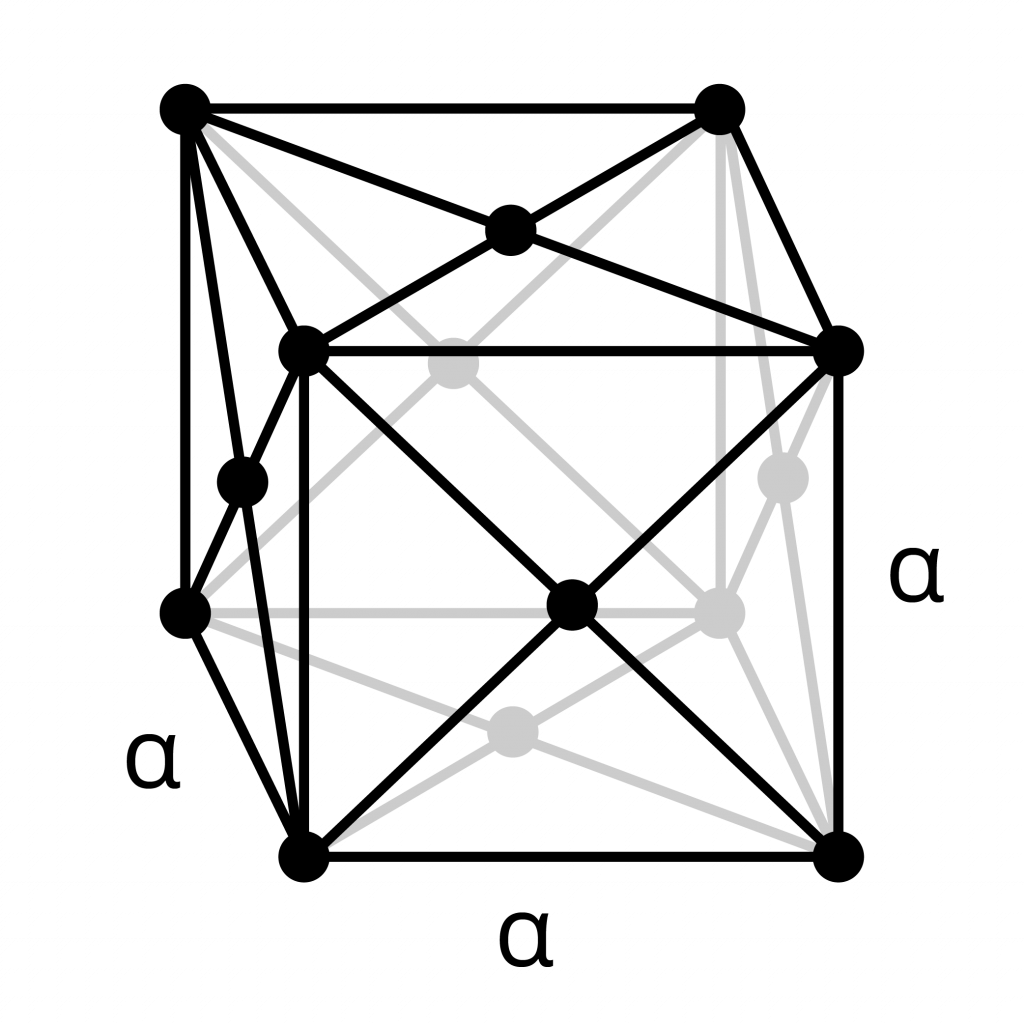
- The face-centered cubic structure has atoms at each corner of the cell and additionally an atom in the center of each face of the cube.
- The “face” centered atoms are shared only with two cells, and so each contribute 1/2 an atom’s worth.
- The unit cell contains 4 atoms total:
8 atoms x 1/8 share for the corner atoms, and 6 atoms x 1/2 share for the face-centered atoms. - Gamma-iron (austenite) is an FCC metal.
Steel, without nickel or manganese, achieves a stable face-centered cubic (FCC) structure between 1,674—2,541°F. At these temperatures, carbon in the steel permeates each cell.
However, this steel, cooled in a regular (unquenched) fashion, will become ferritic and body-centered cubic (BCC). It will not maintain the FCC structure.
BCC lattices are more vulnerable to some types of mechanical strain than more densely-packed FCC structures. They don’t have the same number of atoms in each cell holding the lattice together. Keeping the FCC structure even at room temperatures helps maintain its extra strength. This is usually done with extra elements added to the alloy.
Microstructures of ferritic, austenitic, martensitic, and duplex steels
Ferritic steel is a common BCC steel. It becomes brittle at cryogenic temperatures, loses strength quickly in elevated temperatures, and is magnetic. These properties are due to the body-centered cubic (BCC) form.
Within each “loosely” packed BCC cell, not all electrons are able to find and pair with electrons of the opposite spin. It is these unattached electrons that create the magnetism of the ferritic steel. With only two atoms adding strength to each cell, ferritic steel is also easier to break, especially in hot or cold environments.
Austenitic steel is FCC at room temperature due to an addition of nickel in the alloy. Austenitic steel is more ductile than FCC, even at cryogenic temperatures. It has more heat-strength. It is also not magnetic. These properties are due to its face-centered (FCC) form.
All lattices have “slip systems,” or lines of shear, where the lattice can slide when struck without the cells being ripped apart. Cubic lattices have lots of symmetry and therefore more slip planes. Perhaps counterintuitively, the more densely packed FCC crystal has more lines of shear than the loosely packed BCC crystals. Densely packed crystals slide more easily past each other. Each cell has more atomic weight and strength and holds together more easily.
Plastic deformation at the micro level supports the material’s ductility at the macro level. This is why there is a wider range of resilience in face-centered cubic structures. Ferritic structures are more likely to shatter on impact, or fracture when stretched, especially in challenging environments.
Austenitic stainless steels are the only stainless types that do not become brittle and easily fractured in cryogenic applications. Austenitic steel keeps most of its toughness and elongation even below -292°F. Low-temperature embrittlement is characteristic of ferritic and duplex steels. After a transition temperature they become likely to shatter under stress.
Martensitic steels are another type of steel with a very different type of grain at the surface. These steels do not have a simple cubic microstructure. Martensite is formed by quenching: a rapid cooling of the surface. The environmental shock causes the lattice to heave as it freezes. Martensitic microstructures are under strain, in a body-centered tetragonal shape, and do not line up evenly. This allows martensitic surfaces to be harder, but they are also more brittle, even at room temperature.
Duplex steels are a relatively new addition to the varieties of stainless steels. These steels have a blend of microstructures. Interleaved layers of ferrite and austenite give the final material properties of both. The lower percentages of nickel and/or manganese needed for duplex stainless lowers the cost compared to austenitic stainless.

Care and maintenance of stainless steel
Although stainless steel is rust-resistant, it is not impervious. Its corrosion resistance is based on its passivation layer, which can be disturbed chemically. Salts, acids, scratches that hold moisture, and iron deposits can cause stainless steel to become vulnerable to rust.
Care must be taken when installing stainless: steel tools can change the surface chemistry of the steel by leaving behind iron deposits that make the surface vulnerable. Any place that has come into contact with steel should be cleaned. Deep scratches that could hold moisture should be avoided.
Maintenance of stainless surfaces is not difficult but should be undertaken regularly if the steel is exposed to bumps, scratches, salt, iron, or other chemicals. Outdoor site furniture should be attended to twice per year.
The way to clean stainless steel depends on the type of issue at hand. Different strategies are necessary for different types of marks. Our in-depth cleaning post describes steps for discoloration, rust, grease, fingerprints, cement, or limestone. It is good to deal with corrosion quickly. When caught early, WD-40 or another lubricant may be all that is necessary to remove rust.
With proper maintenance and care, the properties of stainless steel that make it so attractive—steel’s toughness wedded to chromium’s corrosion resistance and luster—can continue to be a stress-free asset for years.
For more information on stainless steel, or to request a quote for a custom project, please contact us.












