From the casting furnace into the mold
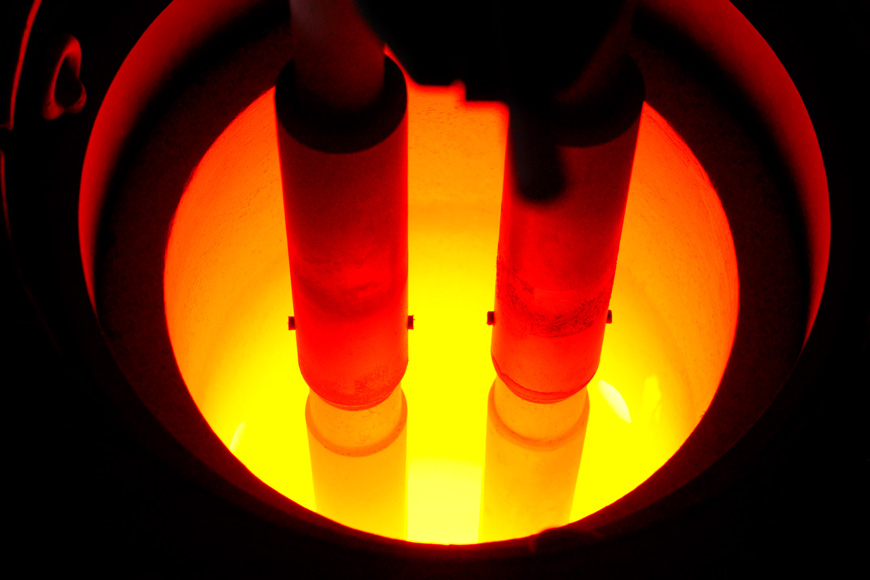
Foundries are dramatic. Huge furnaces, glowing with heat, transform chunks of metal into flowing fiery liquids. When ready, their contents are poured into waiting ladles amid a shower of sparks. Workers guide the flow of metal from furnace to mold behind heat shields, guarding against the dangers of the temperature and materials. The foundry floor is where design becomes actual, in an extraordinary process that creates everyday objects. Innovations in creating and sustaining the temperatures required for different alloys is part of the evolution of metallurgy. The work of melting and pouring metal looks like scenes out of the history books, but it is where some of the most interesting science is happening.
The manufacture of cast metal relies on furnaces that can get hot enough to bring metals to a liquid state. The first smelting of ores in human history were lead and tin: these soft metals can be melted in the heat of the cookfire. To create stronger metals metallurgists needed something more than wood flame.
High heat and human advancement
The Bronze Age was reliant on the strength of copper. Copper may have first been smelted accidentally in a pottery kiln, which runs at least 200°C hotter than a campfire. Lack of written record from that time makes it hard to be sure. During the Bronze Age, kiln-like furnaces were used to extract various elemental components out of rock which melted at different temperatures, with copper being the highest prize for making good brass and bronze.
There is evidence of humans using iron before the Iron Age. However, these items were made of worked iron that had literally fallen from the sky—meteoric iron is in a relatively pure form compared to terrestrial iron. It could be heated and worked as it was found. The true Iron Age started, however, when people figured out how to extract useful iron from ores, and that requires melting to soft, taffy-like, near-liquid, or liquid states. This advance came to different parts of the globe at different times but involved the invention of bloomery furnaces and a slow building of the knowledge of ferrous metallurgy. Bloomery furnaces allow iron to be hot enough to be worked towards purity, rather than bringing metal to the molten state, but they helped with the slow discovery of iron chemistry. It is one thing to melt iron: to make a useable strong metal the right addition of carbon is needed, and bloomery furnaces were carbon fuel reliant. When furnaces got hot enough to melt iron, metallurgists also needed to develop their understanding of fluxes, which are additives used to help purify the final metal by preventing oxide formation.
Advancements brought by innovative combination of furnace and chemistry has continued throughout human history. Metallurgical advance obviously ushered in the Bronze and Iron ages based on their name. The Industrial Revolution entered us into the Steel Age. The combustion engine, railways, and modern building practices would not be possible without an important advance called the Bessemer process, which bubbles oxygen through molten steel allowing for hotter temperatures and quicker production times, allowing for the mass production of quality steel.
Melting vs. smelting
Smelting is the process of removing a metal element from mined ores. Most metals are found as veins in rocks, or as parts of other elements. Smelting is the first step of extraction. Melting is what is done with metal alloys or pure metals. Scrap is melted, ore is smelted. Pig iron is the rough iron ingots created from iron ore smelting.
Blast furnaces
Blast furnaces, which are very tall furnaces injected with pressurized gasses, are used for smelting. Blast furnaces mostly produce ingots of an intermediate alloy, like pig iron. These ingots are then shipped to foundries involved in manufacturing.
Manufacturing foundries take alloys and additives and melt them to make specific grades of cast metal in other types of melting furnaces.

Types of foundry melting furnaces
Traditionally, cupola and crucible furnaces were the most common ways to forge metals for casting; in the modern day, electric arc and induction furnaces are common.
Crucible furnaces
Crucible furnaces are the most basic form of metal furnace. A crucible is a vessel made of material that can handle incredibly high temperatures, often made of ceramic or other refractory material. It is placed into the source of heat like a pot might sit in a fire. The crucible is filled, or charged, with metal and additives. In the modern era, crucible furnaces are still in use by jewelry makers, backyard hobbyists, some non-ferrous foundries, and foundries doing very small batch work. Crucibles can range from a very small cup where metals are melted by blowtorch, like might be done at a jeweler’s, to large vessels that contain 50lbs of material. Larger crucibles are often put inside a kiln-like furnace and can be lifted-out for pouring, or have material ladled off the top.
Cupola furnaces
Cupola furnaces are long, chimney-like, and filled with coal-coke and other additives. The fuel inside the cupola is lit, and when the furnace is sufficiently hot, pig and scrap iron is added directly. The process of melting the iron around the coke and additives adds carbon and other elements and produces different grades of iron and steel. Cupola furnaces are no longer usually used in production, as electric arc and induction methods are more efficient at producing the needed heat. However, there are some places where tradition keep the cupola furnaces running, like in this video of Da Shu Hua, where Chinese foundry workers throw molten iron against a wall to create dramatic sparks to welcome in the New Year.
Electric arc furnaces
(EAFs) came into use in the late 1800s. Electrodes run electrical current through the metal inside the furnace, which is more effective than adding external heat when melting high volumes at one time. A large EAF used in steel production can hold up to 400 tons. A “charge” of this steel is often made of heavy iron like slabs and beams, shredded scrap from cars and other recycling, and pig iron ingots from a smelter.
After the tank is filled, electrodes are placed into the metal, and an arc of electricity passes between them. As the metal begins to melt, the electrodes may be pushed farther into the mix or pulled apart to create a larger arc. Heat and oxygen might be added to speed the process. As molten metal starts to form, the voltage can be turned up, as the slag created on top of the metal acts like a protective blanket for the roof and other components of the EAF.
When everything is melted, the whole furnace is tilted, to discharge the liquid metal to a ladle below. Sometimes the ladles themselves can be smaller EAF furnaces, tasked with keeping the metal hot before pouring.
Induction furnaces
work with magnetic fields rather than with electrical arcs. Metal is charged into a crucible surrounded by a powerful electromagnet made of coiled copper. When the induction furnace is turned on, the coil creates a rapidly reversing magnetic field by the introduction of an alternating current. As the metal melts, the electromagnet creates eddies within the liquid that self-stir the material. The heat in an induction furnace is created by the excitation of the molecules in the iron itself, meaning that whatever goes into the crucible is exactly what comes out: there is no addition of oxygen or other gasses to the system. This means fewer variables to control during melting, but it also means that an induction furnace cannot be used to refine steel. What goes in, comes out. Like an EAF furnace, induction furnaces often discharge by tilting into ladles below.
Induction furnaces are very common and are simple to operate when given high quality input. Common models can produce 65 tons of steel at each charge.

All furnaces on the foundry floor face a fatal enemy: steam. Water, even in small amounts, can cause splashing or explosions, and so all scrap and ferroalloys, as well as every tool used in production, must be dry before use. Scrap metal must not have any closed areas in which water or vapor may have been trapped. Even the tools used by the foundry workers must be free of condensation or moisture. Many foundries have a drying oven to make sure that scrap and tools are bone dry before anything touches the casting furnace.
Casting ladles
After metal is melted, it must be introduced to the mold. In smaller foundries, this may all happen in one stage: a tilting or lift-out crucible may take metal from the furnace to the sand. However, this is impractical when a furnace holds many tons of metal. Typically, in ferrous manufacturing, ladles transfer smaller portions of the melt from the main furnace.

In these systems, a ladle may bring metal straight to the mold. However, a transfer ladle might take the liquid to a holding tank or secondary furnace. Treatment ladles are another available type, used to break the melt into portions, like a baker might separate a basic dough to use it as the base for other recipes. For example, liquid cast iron may have agents added in the treatment ladle to make the carbon within it spherical in shape, rather than flaked, creating a more malleable metal called ductile iron.
Ladles can be very small and lifted by foundry workers or they can hold many tons of metal and need mechanical support. The largest ladles are moved through a foundry by ladle-car or by an overhead crane or track system.
Ladles of all sorts are designed to protect the worker from splash, flames, or sparks while pouring. Some ladles pour over the top lip, or a pour spout, and need to be tilted: these often have gears that allow the foundry worker to carefully control the rate of pouring. Other ladles have their pouring spout at the bottom of the bucket and the pour is controlled by removing and replacing a plug.
Mixing alloys
Metal alloys are made of mixtures of elements which are standardized by a formula that specifies the percentages of each type as well as the steps taken in its manufacture. The melting furnaces and treatment ladles of a foundry are where these alloy types are created for castings.
Foundries often specialize in either ferrous alloys, which contain iron, or specific non-ferrous alloys, like precious metals, copper-based, or aluminum-based alloys.
Ferrous alloys sort into iron and steel. Cast iron alloys include grey iron, which includes silicon, and ductile iron, which has a type of spherical carbon. Grades of cast steel are defined by percentages of carbon and other additives in the mix. Stainless steel is a steel that includes chromium, to help prevent rust through passivation.
Non-ferrous alloys include all other metals, so it is not surprising there is further specialization in non-ferrous foundries. Some places specialize in zinc, some in aluminum; others work primarily with copper-based alloys like brass and bronze. However, there is crossover. If a particular foundry works with both bronze and aluminum, for example, they will likely specialize in certain grades of each.
Whatever alloys a foundry works with, the premise of making molten metal and casting into voids to shape it is the same. An idea becomes actual the moment that metal flows into a mold.












