Why choose galvanized steel over stainless steel?

We say we’re “galvanized” when something spurs us to action. So what does it mean when we use the term galvanized steel or metal?
It turns out, it’s all about the shock.
Ferrous metals contain iron and include 90% of the world’s metal manufacturing. Iron is dense, strong (when mixed with carbon to become steel), plentiful, and easy to refine. These properties make it the most important metal we have for industry and building.
However, iron and many of its alloys are also highly prone to rust when exposed to air and water. Protecting against corrosion is part of designing ferrous metal. Sometimes, this protection can come from the type of alloy; stainless steels, for example, have chromium and other elements for chemical protection against corrosion. Yet these additions can change mechanical properties. They can also be expensive. Sometimes, a sealant is used, like powder coating, paint, or oil treatments.
Galvanized steel is even more common, but it’s less understood. How do you make galvanized steel? Does it spur ferrous metals to action? Does galvanized steel rust? When do you use galvanized steel vs. aluminum vs. stainless steel?
What is galvanized steel?
Galvanization is a process in which zinc coatings are applied to steel or iron, creating a barrier that protects the ferrous metal from corrosion. The layer of zinc works physically by blocking water and air from reaching the steel’s surface, and chemically by offering cathodic protection. This protection is electro-chemical … like the metaphorical “galvanization” that excites us into action. The word comes from the name of 18th century scientist Luigi Galvani, who was a pioneer in bioelectromagnetics.
Galvanic – relating to electric currents created by chemical reaction
Galvanization is usually done at high heat. Although a zinc layer can be added through painting or electroplating, either of these only create a thin layer – a shiny surface only 3 microns thick. A topical application like this is vulnerable in outdoor applications where corrosion resistance is important.
True galvanization happens at high heat, and relies on the chemical interaction between zinc, oxygen, and carbon dioxide. The three react at temperature to create a dark grey zinc carbonate layer that is typically 50 microns thick and much more durable in outdoor locations.
Does Galvanized steel rust?
You may be asking yourself, “does galvanized steel rust?” The answer is yes…to an extent, and based on the conditions. The extent to which galvanized steel rusts is based on the thickness of the protective zinc coating (which we’ll talk about in more detail below) and the type of corrosive environment.
What conditions does galvanized steel rust in? The most common ones include high humidity, wet or soaked environments, salt in the air or water, moss, and acids. But, galvanization also provides good resistance to contact with concrete, mortar, lead, tin, zinc, and aluminum.
Galvanization helps to prolong the fight against rust build-up. It relies on the oxidation of zinc to protect against the oxidation of steel or iron. With the right conditions, galvanized steel resists rusting for up to 50 years. Rust, or the oxidation of ferrous metals, is halted. Yet oxidation is still occurring in the zinc (it’s just that this is not known as rust.)
When the zinc is finally all converted by the oxidation, it will no longer protect and the steel will get munched.

Hot-dip galvanization
Hot-dip galvanization is the most common form of zinc galvanizing. In this method, steel or iron parts are cleaned of debris and mill scale and then are lowered into a zinc or zinc-alloy bath at a temperature near 840°F. The steel is left in the bath until it comes up to the same temperature, after which it is lifted and cooled. This cooling can be done quickly or slowly, depending on the intended look of the final product.
Galvannealing is the process of galvanizing in a zinc bath that has an addition of aluminum. While the metal is still hot, the entire product is heat treated, causing the formation of several strata of zinc-iron alloys. The addition of aluminum and the heat treatment after gives the resulting steel better weldability. Galvannealed steel will generally develop a reddish-rust-like patina but not corrode the way unprotected ferrous alloys corrode.
Dry galvanizing
A less common method for zinc galvanization is dry galvanizing. This process is also known as Sherardizing, named for the metallurgist Sherard Cowper-Coles, who developed the method. In this technique, small steel parts are heated in a closed rotating drum, along with zinc dust and sand, until the temperature and tumble cause the zinc to fuse chemically to the surface of the steel. The part is then quenched. It is often used for small parts and ones with inner surfaces that might be unreached by hot-dip.

Spangle
One of the traditionally recognizable features of galvanized steel is the spangle on its surface. All metal freezes in crystalline forms, like a series of snowflakes packed tightly together. For most metals, this crystalline grain is too small to see, or the grain boundaries are not obvious without etching. Zinc-alloy galvanization allows people to see the crystallization pattern that forms during the freezing of the metal.
Spangle shape and size can give a hint to the cooling conditions after hot-dip galvanizing. The more slowly the surface freezes, the larger the crystal grains, often showing as feather- or leaf-like shapes. A quick quenching to colder temperatures can lead to smaller, more regular, or boxier grain.
Much of the most eye-catching spangle is often created with small amounts of lead or tin in the zinc alloy. These additions allow the dendrite-like creation of the grains—the process of freezing that encourages feather-like shapes stretch across the surface. The development of lead-free galvanization has created zinc coatings that freeze from the steel up. These lead-free alloys may create rounder spangles that can be smaller than .5mm across. The interplay between alloy and freezing conditions means that a skilled galvanizer can influence the size and shape of spangle in the final product.

Galvanic corrosion and a sacrificial anodes
The galvanization process is not just a matter of creating a shell of zinc around the metal to create a physical barrier. If it were, other metals might be used. Rather, zinc protects through a chemical process called “cathodic protection.”
When two metals with very dissimilar electrical potentials are placed together in an electrolyte bath, they begin to act as an anode and cathode in an electrochemical reaction, creating a current. The most “active” of the two metals, the anode, will corrode more quickly than it would if it were alone, because it offers up electrons. The electron-stripped molecules within this anode are chemically unstable, and seek to form stable molecules with chemicals in the environment, producing oxides and other products of corrosion. Batteries use this form of galvanic corrosion to generate current.
Metals are classed by their reactivity in an anodic index. Gold, the most inert or passive of the metals, is used as the reference material. All other metals are given a number that represents their voltage when in an electrolyte bath with gold. This index is the basis for the galvanic scale, which sorts metals from most passive to most active:
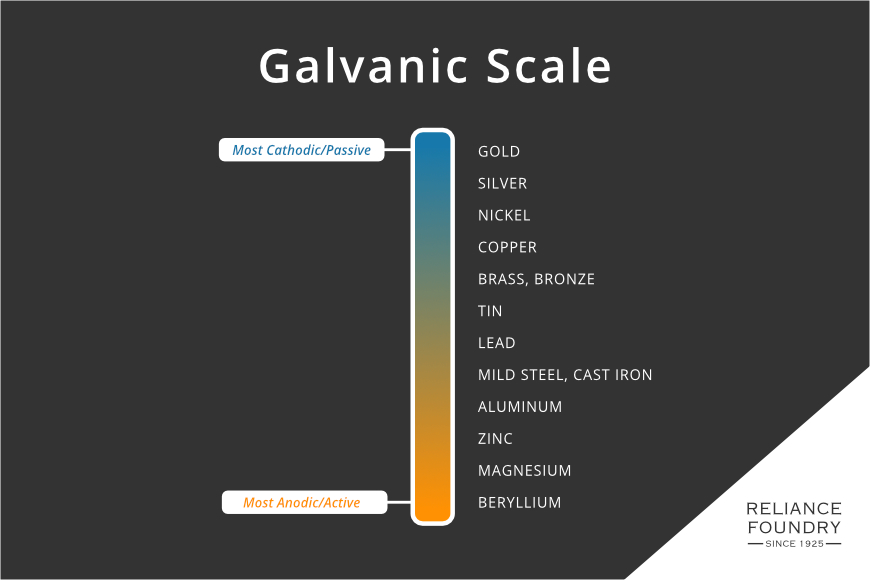
In galvanization, zinc, an anodic metal, covers steel or iron. It works as a sealant, yet if there are any pin-holes in the covering, the electrochemical sacrifice of zinc means that it will corrode first, as the more active of the two metals. This property of a more anodic metal protecting a more cathodic one is used purposefully on boat hulls, which, submerged in sea water, are at risk of corrosion. These sacrificial anodes, also just called zincs, are usually bolted to a hull. Often, zinc parts are included in a design of a propeller or other device.
Zinc sacrifices quickly, but not too quickly, in the presence of iron. Looking at the galvanic scale, it is clear aluminum, magnesium, and beryllium might also be “more active” and therefore protective. However, aluminum is harder to bond to steel, more expensive, and is too close on the anodic index to function as well as a sacrificial anode. On the other side, magnesium would dissolve much faster, and additionally may be more reactive in many environments. Zinc also has the benefit of being protective against organic or bacterial breakdown. Beryllium is rare, expensive, and very unstable.

Finishing galvanized steel
White-rust can form on galvanized steel as the zinc breaks down into zinc hydroxide. This “rust” is indeed the product of zinc corrosion, but in normal conditions it happens at a much slower rate than unprotected steel oxidizes. In order to help slow this process even further, sometimes the galvanized metal is oiled, or occasionally chromium passivated, to create a final sealing layer. This oily or passivated metal cannot be painted or otherwise finished, but in many climates will look good for decades and stay structurally sound for even longer. Galvanized steel is useful for oiled working parts that might see paint quickly worn away.
In applications where the aesthetic of paint or powder-coat is preferred, galvanized steel can be left uncoated. Yet most often an item is either galvanized or painted, not both, to maintain the cost-savings of each approach.
Galvanized steel vs aluminum vs stainless steel
Galvanization is a cost-effective way to help protect metal that will be exposed to potentially corrosive elements. Aluminum and stainless steel are also used for applications that need strong, corrosion-resistant metal. They are better choices for cookware, utensils, and health-care, as too much zinc can be toxic, and both acids and heat might mobilize the zinc. In places where galvanized steel’s dull grey spangle is not ideal, they present a shinier, more adaptable aesthetic.
Aluminum and stainless each cost more, and their mechanical properties are not identical to steel. For applications where mechanical properties, aesthetic, or metal weight are important, more expensive materials might be the optimal choice. In other places, where standard steel’s functionality is desired, the galvanization process is ideal for weatherproofing at low cost.












